Details of the Drug
General Information of Drug (ID: DMQ9RBV)
| Drug Name |
Aminopterin
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
APGA; Aminopteridine; Aminopterine; Aminopterinum; Aminotrexate; Pteramina; Pteramina [Czech]; A 1784; A-7170;A-Ninopterin; ENT-26079; Kyselina 4-aminolistova; Kyselina 4-aminolistova [Czech]; Kyselina 4-aminopteroylglutamova; Kyselina 4-aminopteroylglutamova [Czech]; L-Glutamic acid, N-[4-[[(2,4-diamino-6-pteridinyl)methyl]amino]benzoyl]; N-(1-((2,4-Diamino-6-pteridinylmethyl)amino)benzoyl)glutaminsaeure; N-[(4-{[(2,4-diaminopteridin-6-yl)methyl]amino}phenyl)carbonyl]glutamic acid; N-(4-{[(2,4-diaminopteridin-6-yl)methyl]amino}benzoyl)-L-glutamic acid; N-(4-(((2,4-Diamino-6-pteridinyl)methyl)amino)benzoyl)-L-glutamic acid; Kyselina N-(p-((2,4-diamino-6-pteridinyl)methyl)benzoyl)-L(+)-glutamova; Kyselina N-(p-((2,4-diamino-6-pteridinyl)methyl)benzoyl)-L(+)-glutamova [Czech]; Kyselina N-(p-((2,4-diamino-6-pteridinylmethyl)amino)benzoyl)-L(+)-glutamova; Kyselina N-(p-((2,4-diamino-6-pteridinylmethyl)amino)benzoyl)-L(+)-glutamova [Czech]; N-(4-(((2,4-Diamino-6-pteridyl)-methyl)amino)benzoyl)-L-glutamic acid; (2R)-2-[[4-[(2,4-diaminopteridin-6-yl)methylamino]benzoyl]amino]pentanedioic acid; (2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methylamino]benzoyl]amino]pentanedioic acid; 2-[[4-[(2,4-diaminopteridin-6-yl)methylamino]benzoyl]amino]pentanedioic acid; 4'-Amino-folsaeure; 4'-Desoxy-4'-aminofolsaeure; 4-Amino pteroylglutamic acid; 4-Amino-4-deoxypteroylglutamate; 4-Amino-4-desoxy-pteroylglutaminsaeure; 4-Amino-PGA; 4-Aminofolic acid; 4-Aminopteroyl-<R>glutamic acid; 4-Aminopteroyl-L-glutamic acid; 4-Aminopteroyl-glutamic acid; 4-Aminopteroylglutamic acid
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Anticancer Agents
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
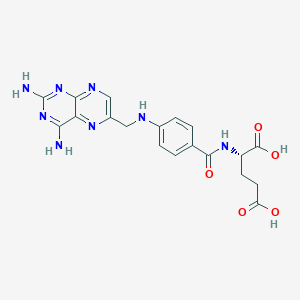 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 440.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 9 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 6 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 12 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | leukaemia | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 2A60-2B33 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
